
It is important to talk about fowl typhoid in poultry egg and meat production. Firstly, because there is certain ignorance around the pathogen that causes “fowl typhoid” since it is part of an extensive bacterial group with a complex taxonomical system. Secondly, because isolating it is somewhat complicated due to its biological behaviour. And thirdly, because any talk about Salmonellosis immediately puts consumers and producers on alert due to the zoonotic and economic implications related to product losses and stigmas attached to poultry product brands.
Aetiological agent, transmission and immunity
The aetiological agent is a biovar of the Salmonella enterica genus called S. Gallinarum or S. pullorum, which is host-dependent and particularly affects fowl of the Gallus gallus genus. The pathogen is therefore classified as host-specific and is responsible for causing high levels of mortality and considerable egg and meat production losses in susceptible bird groups.
Transmission of S. gallinarum or S. pullorum can occur three different ways: vertically, para-vertically or horizontally. It is an intracellular pathogen targeting cell groups in the reticuloendothelial system, liver and kidney cells and reproductive system. It can lay low in these cell groups for long period of times without clinically manifesting (Manon R., et. al., 2014). Under stressful conditions or upon reaching sexual maturity, S. gallinarum takes on clinical relevance, leading to the onset of symptoms and elevated mortality (Castro Eguíluz, A. D. 2013).
The immune system’s mechanisms of avoidance are mediated by the coding and production of chemical messengers that successfully block, among others, the cellular machinery of the attacked cells, including the immune response, particularly that of the cellular order. It seems that antibody-mediated immunity is not as effective at controlling the disease (Rodríguez Hernández R., 2015).
Protection against S. gallinarum
The need to improve protection against S. gallinarum by seeking to reduce the susceptibility of the birds and slow down the speed of transmission has made it possible to leverage multiple immunological strategies, including the use of a rough vaccine strain known for more than sixty years and universally referred to as 9R. However, this strain confers short-term immunity (Singh B. R., et. al., 2005) and its ability to persist in immunised birds facilitates the presence of concomitant infections with wild strains. This makes it a candidate solely for use in emergency situations in expensive birds not used for reproduction and where outbreaks of “fowl typhoid” are acute or peracute, and several doses may be needed until the affected birds exit the production cycle.
Upon performing a comparative analysis via rapid plate agglutination of thirty-one (31) groups of brown laying hens located in an automatic cage system with a density of 8.9 birds per cage, sourced from breeding hens from outside the farms, located in different parts of the country and born on different dates, it can be concluded that the mortality in the evaluated groups, despite not being correlated with the positive blood cultures in the test, does show that the older groups have higher weekly mortality (Figure 1). This was confirmed in the linear regression models where mortality was evaluated as a dependent variable of the time variable.
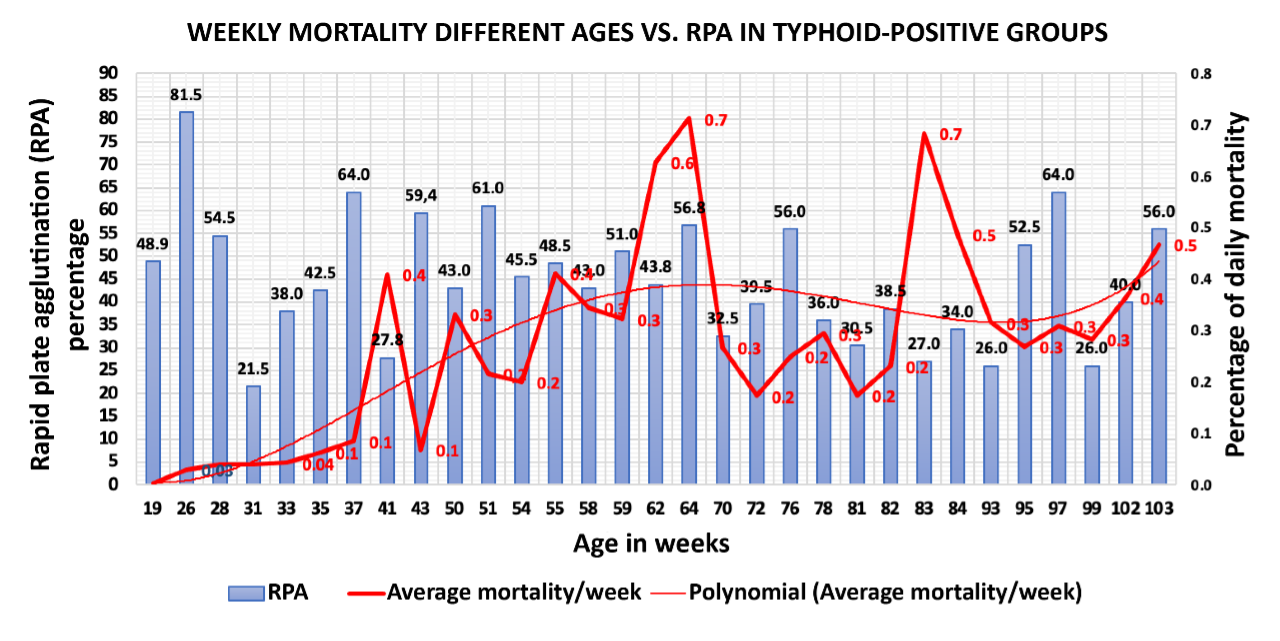
Figure 1. Mortality behaviour in groups of different ages The polynomial trendline shows the increase in bird deaths with no direct relationship with the percentage of fowl typhoid positivity via the rapid plate agglutination technique using antigen supplied by Charles River
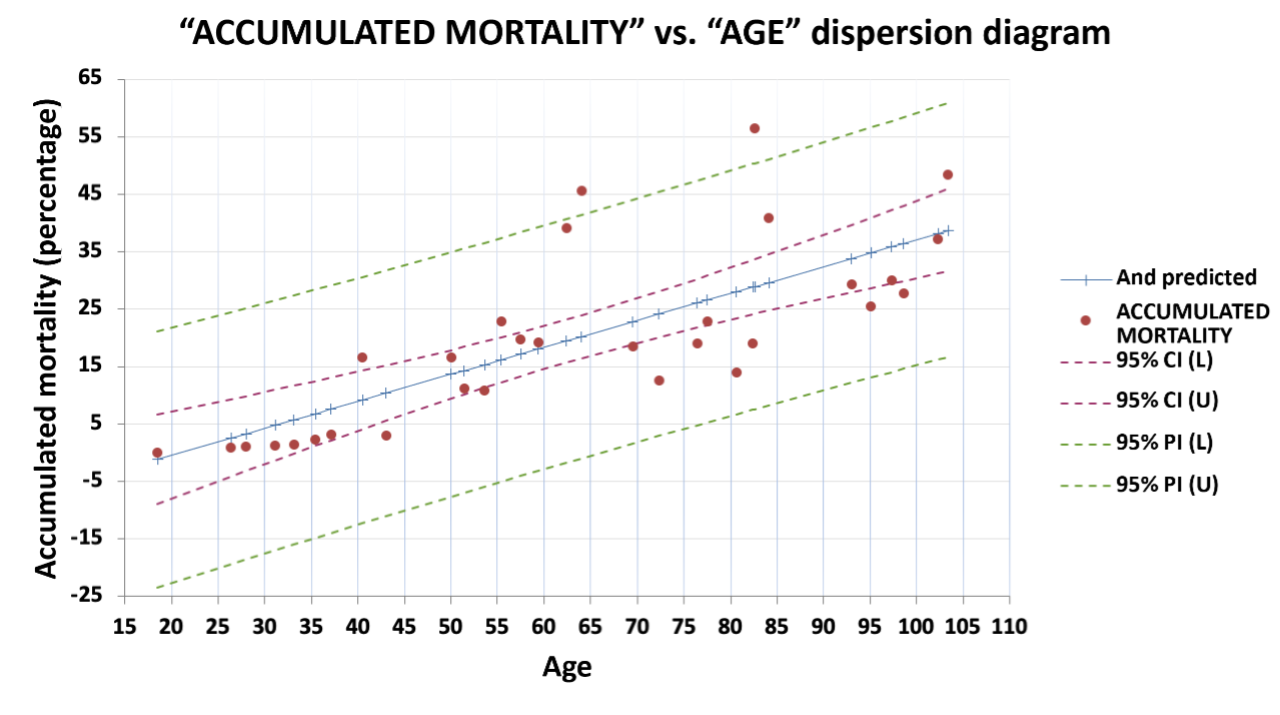
Figure 2. Predicted behaviour of fowl mortality from week 20 to 110. The regression line shows the evolution of mortality in “Fowl Typhoid”-positive groups
A meta-analysis comparing different immunisation plans for “Fowl Typhoid”, which included approximately three hundred groups vaccinated against Salmonella enteritidis and gallinarum, offered the opportunity to statistically compare the performance of the primary zootechnical variables of each of the studied groups. The vaccine regimens against salmonellosis were as follows:
- A combination regimen using three doses of a “metabolic mutant” live vaccine (PRIMUN SALMONELLA E) by the oral route plus two doses of an inactivated vaccine containing S. enteritidis and S. gallinarum (SALMONELL BAC);
- A regimen based on two and/or three doses of the “rough live 9 R” vaccine by subcutaneous injection.
- A third regimen which only involved two doses of the S. enteritidis inactivated vaccine by the intramuscular route.
The result obtained from the analysis showed the following:
- The groups vaccinated only with inactivated vaccines behaved the same as the positive groups without any vaccine (data not recorded in this analysis);
- The groups vaccinated with the regimen involving the “metabolic mutant” live vaccine (PRIMUN SALMONELLA E) plus the inactivated vaccine presented statistically significant differences in mortality and therefore in egg production compared to the groups vaccinated with the rough strain 9R vaccine (figure 3).
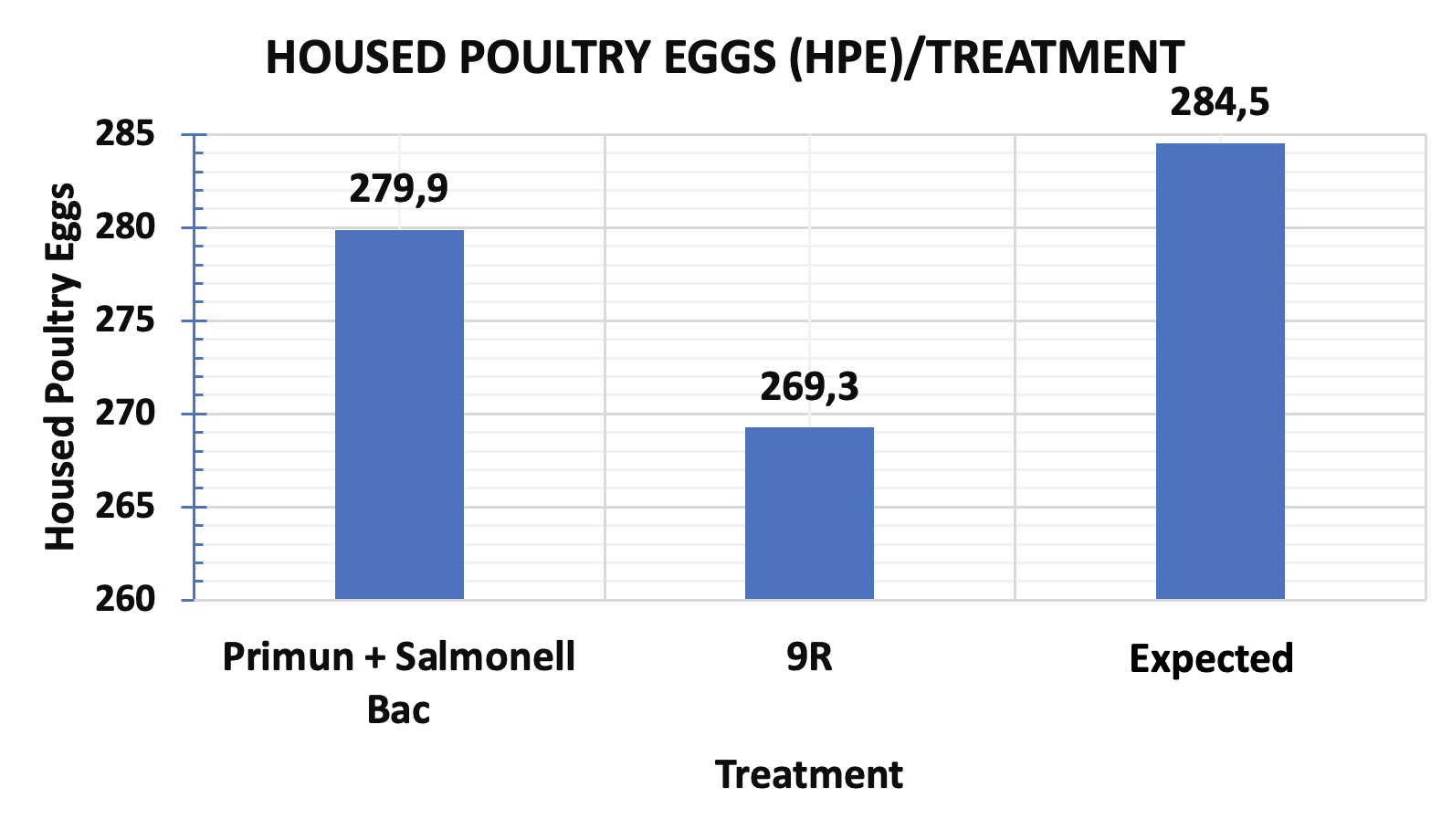
Figure 3. Housed poultry egg production in groups of birds positive for “Fowl Typhoid” vaccinated with “S. gallinarum rough strain” versus a group of birds vaccinated with S. enteritidis “metabolic mutant” (PRIMUN SALMONELLA E) plus the inactivated vaccine with S. enteritidis and S. gallinarum (SALMONELLA BAC)
The results obtained in the meta-analysis made it possible to reach a conclusion on various premises regarding controlling S. gallinarum and/or pullorum, including the following:
- The possibility of vertical or para-vertical transmission from breeding hens to progeny is an important pathway of dissemination.
- It is impossible to determine the infection status of a group without performing an adequate seropositivity analysis.
- Regardless of the positivity percentage of a group, mortality will always be high in groups that are not adequately vaccinated.
- A group that is positive for “Fowl Typhoid” will always be positive.
- A well-designed vaccine regimen is needed to control the disease.
- The immunity conferred by “rough strain 9R” is short-lasting (Singh B. R., et. al., 2005).
- The immunity conferred by a live vaccine containing S. enteritidis metabolic mutant (PRIMUN SALMONELLA E) plus an inactivated vaccine containing S. enteritidis and S. gallinarum is longer-lasting and more consistent. (Wigley P., 2017; Barrow P. A., 2012).
In conclusion, regardless of the individual efficacy of certain vaccine products, zootechnical monitoring of groups that are positive for this disease is very important to determine which programme can help deliver the best results



